electron releasing groups|electron withdrawing groups and donating : Manila Although the full electronic structure of an arene can only be computed using quantum mechanics, the directing effects of . Tingnan ang higit pa Expand your career horizons at the Pintados de Pasi' PESO Job Fair on March 19, 2024. Explore exciting opportunities with top local and overseas employers at CityMall, Passi City. Streamline your experience! Pre-register at the Public Employment Service Office (PESO) located in the OFW Building.
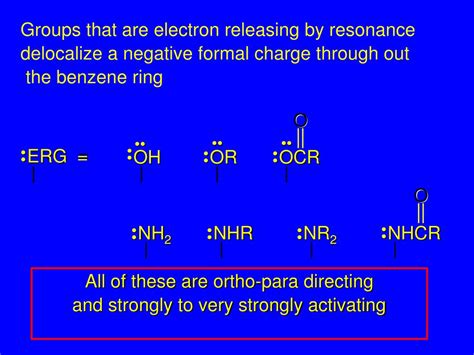
electron releasing groups,In electrophilic aromatic substitution reactions, existing substituent groups on the aromatic ring influence the overall reaction rate or have a directing effect on positional isomer of the products that are formed. An electron donating group (EDG) or electron releasing group (ERG, Z in structural formulas) is an . Tingnan ang higit paElectron donating groups are typically divided into three levels of activating ability (The "extreme" category can be seen as "strong".) Electron withdrawing groups are assigned to similar groupings. Activating substituents . Tingnan ang higit paThere are 2 ortho positions, 2 meta positions and 1 para position on benzene when a group is attached to it. When a group is an ortho / para director with ortho and para . Tingnan ang higit pa• Electrophilic aromatic substitution Tingnan ang higit pa
Although the full electronic structure of an arene can only be computed using quantum mechanics, the directing effects of . Tingnan ang higit paWhen two substituents are already present on the ring, the third substituent's new location is relatively predictable. If the existing substituents reinforce or the. Tingnan ang higit pa
An electron releasing group or ERG (may also be called electron donating groups or EDG's) releases electrons into a reaction center and as such stabilizes electron .
Electron-donating substituents increase the electron density of the aromatic ring, activating it, and increasing the rate of electrophilic substitutions. Electron-withdrawing . Electron donating groups are alkyl groups, phenyl groups or substituents that have a lone pair of electrons on the atom directly bonded to the ring. Electron .
Objectives. After completing this section, you should be able to. account for the basicity and nucleophilicity of amines. explain why amines are more basic than .
Examples of good electron donating groups are groups with lone pairs to donate, such as: The oxygen anion, -O-Alcohol groups, -OH Amine groups, -NH 2 or -NR 2; .

Examples of Electronic Effects. The Inductive Effect. Resonance. The Mesomeric Effect. Electromeric Effect. Hyperconjugation. Inductive Effect. The inductive effect is a .Electron donating group (EDG; electron releasing group; ERG): An atom or group that release electron density to neighboring atoms from itself, usually by resonance or inductive effects.An electron releasing group or ERG (otherwise called electron donating groups or EDG) releases electrons into a reaction center and as such stabilizes electron deficient .Strong electron‐releasing and electron‐withdrawing groups significantly reduced the band gap. Electron‐withdrawing groups red‐shifted the absorption spectra. A reverse .Electron donating group (EDG; electron releasing group; ERG): An atom or group that release electron density to neighboring atoms from itself, usually by resonance or inductive effects. Methyl carbocation Less .
When this center is an electron rich carbanion or an alkoxide anion, the presence of the electron-withdrawing substituent has a stabilizing effect. Similarly, an electron-releasing group (ERG) or electron-donating group (EDG) releases electrons into a reaction center and as such stabilizes electron deficient carbocations.

Remember that, relative to hydrogen, alkyl groups are electron releasing, and that the presence of an electron‑releasing group stabilizes ions carrying a positive charge. Thus, the free energy difference between an alkylamine and an alkylammonium ion is less than the free energy difference between ammonia and an ammonium ion; .
electron releasing groups electron withdrawing groups and donatingRemember that, relative to hydrogen, alkyl groups are electron releasing, and that the presence of an electron‑releasing group stabilizes ions carrying a positive charge. Thus, the free energy difference between an alkylamine and an alkylammonium ion is less than the free energy difference between ammonia and an ammonium ion; .Electron donating group (EDG; electron releasing group; ERG): An atom or group that release electron density to neighboring atoms from itself, usually by resonance or inductive effects. Methyl carbocation Less stable Tert-butyl carbocation More stable: The tert-butyl carbocation is more stable .electron withdrawing groups and donating The second type of inductive effect is designated by the symbol +I and describes the properties of electron-releasing groups or atoms. The positive inductive effect, or +I effect, occurs when a chemical species that tend to release or donate electrons, such as an alkyl group, is added to a carbon chain, causing the charge to be . The electron-withdrawing nature of groups or atoms is called a negative inductive effect. It is indicated by -I. When the substituent can be considered as an electron releasing group based on the resonance structures, the effect is positive +M. The electron-releasing nature of groups or atoms is called a positive inductive effect. The important electron withdrawing groups should be remembered for NEET examination point of view are halogens (F, Cl), nitriles CN, carbonyls RCOR' and nitro groups NO2. . The release of electrons into a reaction centre by an electron releasing group, also known as an ERG or electron donating group (EDG), stabilises .
00:00 General Ideas 00:54 Electron-donating Groups02:30 Examples of Electron-donating Groups06:15 Electron-withdrawing Groups08:07 Examples of Electron-withd. Remember that, relative to hydrogen, alkyl groups are electron releasing, and that the presence of an electron‑releasing group stabilizes ions carrying a positive charge. Thus, the free energy difference between an alkylamine and an alkylammonium ion is less than the free energy difference between ammonia and an ammonium ion; .Applications of Mesomeric Effect. In chemistry, the mesomeric effect, also known as the resonance effect, is a feature of substituents or functional groups in a molecule. The effect is symbolized by the letter ‘M’ and is used to describe the electron-withdrawing or releasing properties of substituents depending on the relevant resonance . 1) a) Consider the inductive effects of the substituents attached to the carboxylic acid. The tert-butyl group is electron-donating which should decrease the acidity of the carboxylic acid. The trimethylammonium substituent is positively charged and can be a powerful electron-withdrawing substituent.If the $$\pi$$ electrons move away from the group and towards the rest of the molecule, the effect is called a +M effect. An example is the donation of electrons from an amino group into a benzene ring, putting $$\delta^{-}$$ charges on the ortho and para positions.Illustrated Glossary of Organic Chemistry. Electron withdrawing group (EWG): An atom or group that draws electron density from neighboring atoms towards itself, usually by resonance or inductive effects. Trifluoro .
Electron releasing groups. Aromatics containing electron releasing groups such as phenols, dim ethyl am in oben 2en e and indole are formylated by 2-ethoxy-l,3-dithiolane in the presence of boron trifluoroetherate, followed by hydrolysis (114). The preformed dithiolanium tetrafluoroborate also undergoes Friedel-Crafts reaction with aromatics .Note that, as do the hydroxyl and amino groups, the halogens have an inductive electron-withdrawing effect and a resonance electron-releasing effect on a benzene ring. The difference in behavior during electrophilic substitutions arises because, with the hydroxyl and amino groups, the resonance effect is much greater than the inductive effect .electron releasing groups An electron releasing group stabilizes the intermediate carbocation by delocalizing [or neutralizing] the positive charge . More stable the carbocation ,faster is the reaction . On the other hand , an electron withdrawing group takes away the electron from an already electron deficient ring and thus , intensifies [increases] the positive charge .Reason (R) Alkyl groups are electron releasing groups. View Solution. Q2. Assertion: 3 + 7 = 9 is a statement. Reason: A sentence that can be judged to be true or false, but not both, is called a statement. (a) Both Assertion and Reason are true and Reason is a correct explanation of Assertion.This kind of electron distribution in unsaturated compounds conjugated with electron-releasing or withdrawing groups or atoms is called mesomeric effect. As shown above, a polarity is induced in compounds due to transfer of electrons through \(\pi\) bonds. This effect is a consequence of resonance and is seen in compounds that contain a double .
electron releasing groups|electron withdrawing groups and donating
PH0 · strongest electron withdrawing group
PH1 · order of electron withdrawing group
PH2 · no2 is electron donating or withdrawing
PH3 · ewg group chemistry
PH4 · electron withdrawing groups list
PH5 · electron withdrawing groups examples
PH6 · electron withdrawing groups and donating
PH7 · electron releasing groups examples
PH8 · Iba pa